Patient Reported Outcomes (PROs) is one of the Trauma MICs cross-cutting themes, Dr Anita Slade has kindly written the insightful piece below to highlight The Centre for Patient Reported Outcomes Research (CPROR) and the importance of Patient Reported Outcome Measures (PROMs).
The Centre for Patient Reported Outcomes Research (CPROR)
The Centre for Patient Reported Outcomes Research is an internationally recognised research centre that aims to ensure best practice in the use of Patient Reported Outcomes (PRO) in clinical trials, practice and research. The centre director Professor Melanie Calvert is Professor of Outcomes Methodology at the University of Birmingham and theme lead for the NIHR Trauma MIC.
Patient Reported Outcome Measures
Patient reported outcome measures (PROMs) are questionnaires completed by patients to provide valuable information on the impact of disease and medical intervention (such as a medical device) on their symptoms and quality of life. PROMs are frequently used to evaluate interventions from the patient perspective in clinical trials, practice and research. A good PROM should reflect the lived experience of the person completing the PROM. Research has shown this helps to deliver quality measurements which reflect the true impact of any interventions. PROMS cover a wide range of areas from specific symptoms like pain to more generic qualities like health-related quality of life. They may be used as a primary, secondary or exploratory outcomes in clinical trials including medical device evaluations.(1)
PROMs and Medical device evaluation
The choice of PROM should reflect expected outcomes from medical device evaluations.(2, 3) A PROM may be used to capture treatment benefits or risks in medical device research. The development and use of PROMs has seen an exponential growth in the last decade. Their importance in clinical trials and medical device evaluation is increasingly being recognised by organisations like the NIHR and FDA. The FDA and increasingly the EMA require evidence to support the use and choice of PROMs in clinical trial protocols, and require this evidence as part of any labelling claim submissions (2). The FDA has also recently brought out a consultation document on the use of PROMs in medical device registrations which closely follows the advice from the current medical labelling claims guidance document.(2, 3)
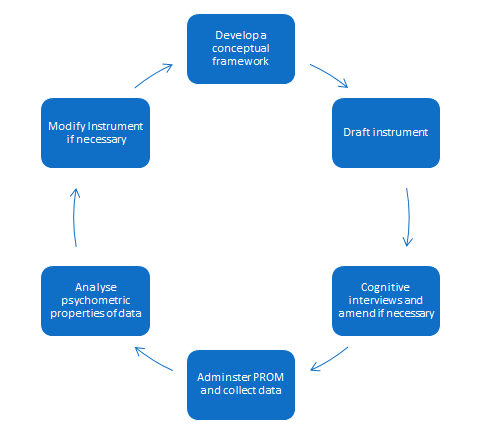
Previous studies have demonstrated that PROMs developed with input from people who have experience of living with the health condition are more sensitive and have superior content validity (4). The new proposed FDA guidelines for medical devices advocate that identification or development of PROMs for use in clinical trials should be underpinned by a conceptual framework with potential participants contributing to that framework (5, 6).
It is important to consider which outcomes are going to be used to evaluate medical devices early on in their development. The quality and choice of PROM should be considered, and needs to reflect expected outcomes and the population being evaluated. If a suitable instrument is not found, a new PROM will need to be developed and this can be a lengthy business. In some situations it can be possible to modify an existing instrument.
Figure 1: Process for developing a PROM following FDA guidelines
The Role of CPROR
CPROR can guide you throughout the process of choosing and evaluating suitable PROMs and help with developing clinical trial protocols, medical device evaluations and grant applications which have a PRO element.
The myriad of PROMs available can be difficult to navigate when deciding on which PROM to use, and new measures are being developed all of the time. The quality of PROMs as measurement instruments can vary particularly if the measure was developed before development guidelines and more rigorous methods of psychometric analysis were used. CPROR has a team of highly qualified researchers and psychometricians who can help you with all aspects of device evaluation using PRO. The team also has developed a free web-based information resource – PROlearn, and also offer CPD training.
If you would like input from the team please contact Dr Anita Slade (a.l.slade@bham.ac.uk) and complete our new business form.
1. Calvert M, Kyte D, Mercieca-Bebber R, Slade AL, Chan A-W, King M, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: The SPIRIT-PRO Extension. JAMA. 2018;319(5):483-94.
2. FDA. Patient reported outcome measures: Use in Medical Product Development to Support Labeling Claims. In: (CDER) CfDEaRFaDA, editor. Silver Spring, MD: US Dept of Health and Human Services Food and Drug Administration; 2009. p. 39.
3. FDA. Principles for Selecting, Developing, Modifying, and Adapting Patient-Reported Outcome Instruments for Use in Medical Device Evaluation USA: Food and Drugs Agency; 2020 [Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/principles-selecting-developing-modifying-and-adapting-patient-reported-outcome-instruments-use.
4. Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-Reported Outcomes to Support Medical Product Labeling Claims: FDA Perspective. Value in Health. 2007;10:S125-S37.
5. FDA. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring, MD: US Department of Health and Human Services, Adminstration FaD; 2009.
6. Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content Validity—Establishing and Reporting the Evidence in Newly Developed Patient-Reported Outcomes (PRO) Instruments for Medical Product Evaluation: ISPOR PRO Good Research Practices Task Force Report: Part 1—Eliciting Concepts for a New PRO Instrument. Value in Health. 2011;14(8):967-77.