We are one of 11 MedTech and In vitro diagnostic Co-operatives (MICs), centres of expertise that focus on clinical areas of high morbidity and unmet need for NHS patients and healthcare technology users.
Our main priority is engagement with Small/Medium Enterprises to deliver improvements in technology for patients; to this end we have worked with over 200 different sized companies both nationally and internationally to develop concepts, support device evaluations and accelerate adoption.
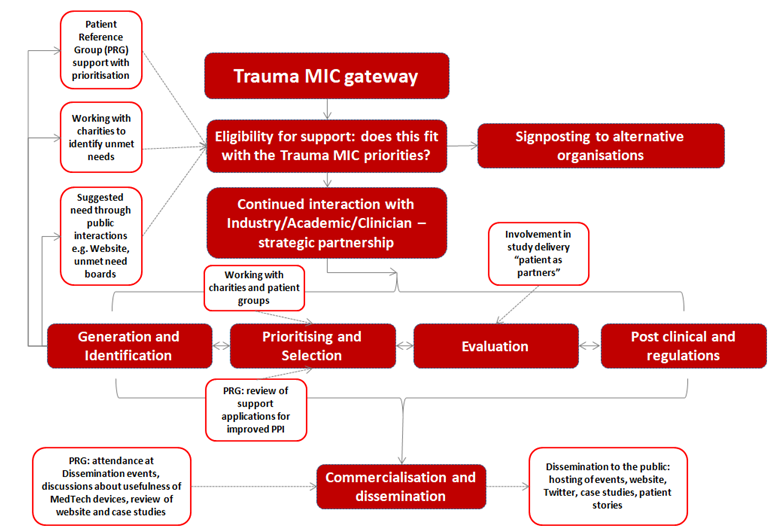
Types of support the NIHR Trauma MIC can offer your company includes:
IDENTIFY the “problem” and requirement for the development of device solutions
- Working with patients, carers, charities and healthcare professionals to identify unmet needs within the trauma field- with the aim to support research in those areas and share learning.
- Regulatory advice for companies wishing to attain CE approval for devices (this is required for devices to be used in a healthcare setting in the EU).
- Prototype development including 3D scanning, CAD design and modelling.
- Usability studies – small scale studies designed to explore how patients/care professionals experience using a new device in order to achieve IEC 62366.
Provide ACCESS to key people, resource and opportunities (Prioritising, Selection and Evaluation)
- ‘Matchmaking’ – finding appropriate resources and clinical/academic staff who would be willing to support projects with an external partner.
- Patient and Public Involvement expertise to gauge patients’ opinions and priorities relating to their condition, advice on public engagement, as well as PPI design support for studies.
- Grant award application support, either by directly applying for funding, collaborating on a project or providing consultation on the application process.
- Support to gain clinical evidence which can be used to substantiate the effectiveness and adoption claim.
SUPPORT Adoption (commercialisation and dissemination of device/healthcare technology)
- Events management for workshops and meetings with external partners to discuss new research opportunities.
- Dissemination of research and findings.
- Advice on market access and adoption.
Map of industry interactions to date:
 Please do not hesitate to contact us you would like further information.
Please do not hesitate to contact us you would like further information.

