Health Economics is a Trauma MIC cross-cutting theme. The Trauma MIC have recently part-funded a new health economist, Luiz Andrade. Luiz has kindly written the below piece to provide some input on methods for early economic evaluation of medical devices still at the design stage.
Background
Firms investing in the development of healthcare devices face a long and complex development pathway. In days gone by, the developer would be motivated purely by fulfilling a need. That is no longer enough, the device must do more than fill a need. Third party payers increasingly determine what may be provided in health services on the basis of cost-effectiveness. NICE fulfils this function for the National Health Service (NHS) in England and Wales. It is therefore prudent for developers and investors to consider early economic analysis to optimise investments and guide R&D decisions. This way, new devices are more likely to succeed in the modern market (1,2).
If it is a blockbuster, the decision to develop a technology can be made without any formal method of evaluation, however such scenarios are rare. In more common scenarios we recommend the practical decision pathway represented by Figure 1.
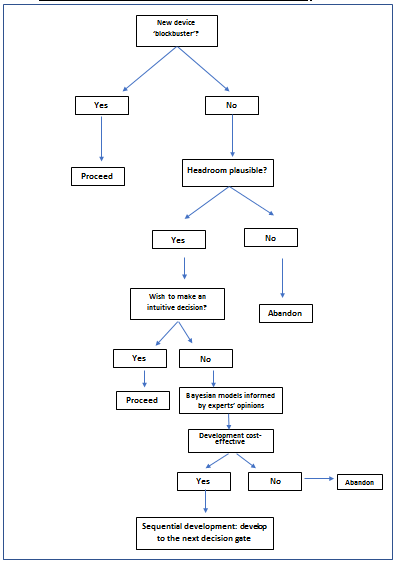
Figure 1: Sequential decision pathway during medical devices development
The headroom method
Formal analysis using health economic evaluation methods can be applied during different phases of the product development process (2,3). The headroom or threshold analysis can be used as an alternative to a full economic model (2,3,4). Instead of asking if the device is cost-effective, we ask how effective the device would have to be to be cost-effective or how much the device could be charged at a given level of effectiveness.
Bayesian probabilities informed by experts’ opinions
If the headroom suggests the device could be cost-effective, then the next step is a formal cost-effectiveness model. Because effectiveness data in the early development stage is scarce, models must be adapted to the lack of evidence regarding effectiveness of the intervention under development (5,6). Expert opinion in the form of Bayesian probability densities can then be used in cost-effectiveness models (1,2,6).
Sequential development
The decision to develop a device does not need to be made once and for all, the development can be an iterative process. In this case the development of each iteration leads to a decision gate; on the basis of cumulative knowledge a stop-go decision can be made. The process is analogous to a value of information decision (7); the decision to drill for oil, obtaining diagnostic information or abandon the site. The option to delay a decision and accumulate more information has a value. This is a fundamental difference between supply and demand side economics as illustrated in Figure 2.
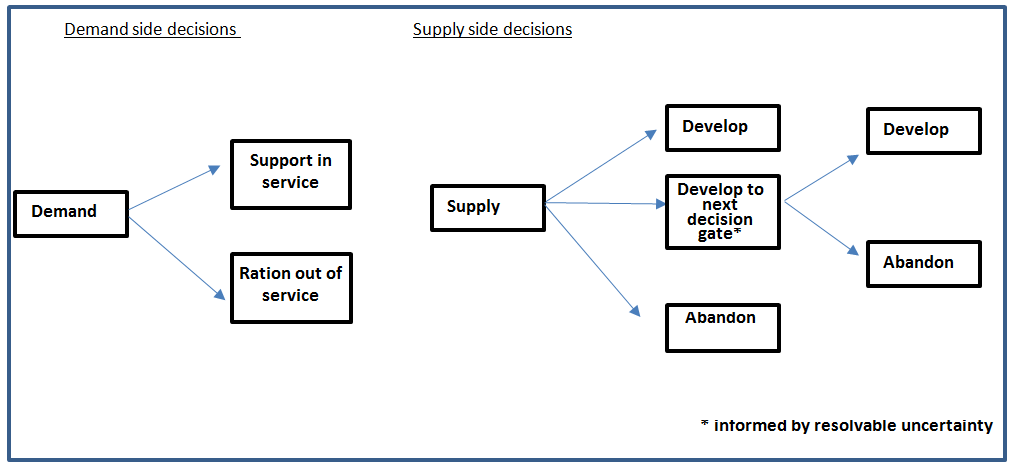
Figure 2: Fundamental difference between supply and demand side economic appraisal.
In conclusion, early health economics methods can help guide development decisions by manufacturers.
References
1 Spiegelhalter, DJ, Myles, JP, Jones, DR, Abrams KR. Bayesian methods in health technology assessment: A review. Health Technol Assess. 2000; 4:1–130
2. Vallejo-Torres L, Steuten LM, Buxton MJ, Girling AJ, Lilford RJ, Young T. Integrating health economics modeling in the product development cycle of medical devices: a Bayesian approach. Int J Technol Assess Health Care. 2008 Fall;24(4):459-64.
3. Cosh, E., Girling, A., Lilford, R. et al. Investing in new medical technologies: A decision framework. J Commer Biotechnol 13, 263–271 (2007).
4. Girling A, Lilford R, Cole A, Young T. Headroom approach to device development: current and future directions. Int J Technol Assess Health Care. 2015;31(5):331-338.
5. Fenwick E, Palmer S, Claxton K, Sculpher M, Abrams K, Sutton A. An Iterative Bayesian Approach to Health Technology Assessment: Application to a Policy of Preoperative Optimization for Patients Undergoing Major Elective Surgery. Medical Decision Making. 2006;26(5):480-496.
6. Love-Koh J. How Useful Are Early Economic Models? Comment on “Problems and Promises of Health Technologies: The Role of Early Health Economic Modelling”. Int J Health Policy Manag. 2020 May 1;9(5):215-217.
7. G. Favato, G. Baio, A. Capone, A. Marcellusi, F.S. Mennini. A novel method to value real options in health care: the case of a multicohort human papillomavirus vaccination strategy Clin Ther, 35 (7) (2012), pp. 904-914.

