PROJECT SUMMARY
The NIHR Trauma Management MedTech Co-operative (NIHR Trauma MIC) collaborated with a UK based company, Braidlock Ltd, on human factors validation testing for their Braidlock® system encompassing line, drain and catheter securement.
CLINICAL NEED
Within the global clinical environment 500 million lines are utilised annually. Over half of these are inadvertently moved or dislodged, negatively impacting patient safety and comfort
THE SOLUTION
Braidlock Ltd work with healthcare professionals to develop modern, cost effective, and safe methods of line securement to improve patient comfort and safety in a cost effective manner. The Braidlock® system also offers a reduced infection risk as it is designed to encounter minimal skin contact, allowing for easier cleaning of the wound site.
HOW WE SUPPORTED
The NIHR Trauma MIC conducted human factors validation (usability) testing to demonstrate the device could be utilised:
- by the intended users without serious use errors or issues
- for the intended device uses
- under the expected use conditions
The components used within this study were:
- Braidlock® Devices for Intercostal Drain fixation
- Braidlock® Devices for Nasogastric Tube fixation
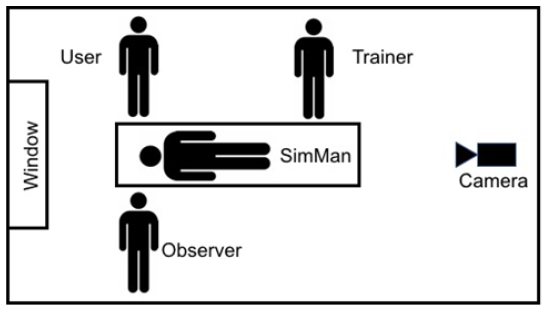
The NIHR Trauma MIC supported by:
- Recruiting the volunteers (a mixture of medical, nursing, and practitioner staff)
- Administering the consent process
- Facilitating the training
- Carrying out filming and observations throughout the study
- Conducting a post-study questionnaire and interview
- Compiling a comprehensive usability summative test report for Braidlock®
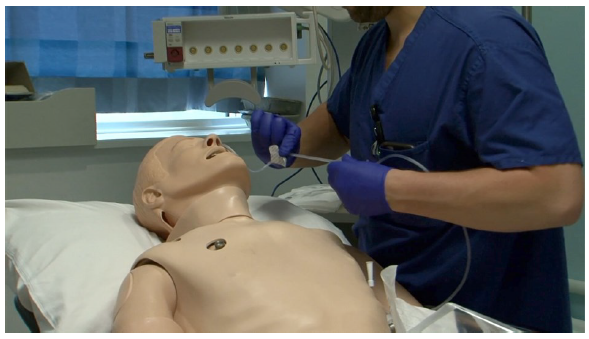
OUTCOME
Feedback reported from the usability testing will help Braidlock Ltd with further product development opportunities. A project plan is being implemented to address the clinical needs highlighted. This is reflected in
two areas:
Firstly, to be able to change the adhesive dressing while the Braidlock® is in situ to extend the usability of the device. This will greatly support clinicians and patients who require longer term catheter / tube securement.
Secondly, clearer product identification and orientation would be beneficial to improve operator use and satisfaction and reduce potential risk of user error.
CONTACT
Web: www.braidlock.com
Mail: info@braidlock.com
Tel: +44 (0) 1292 477159

