Who we are
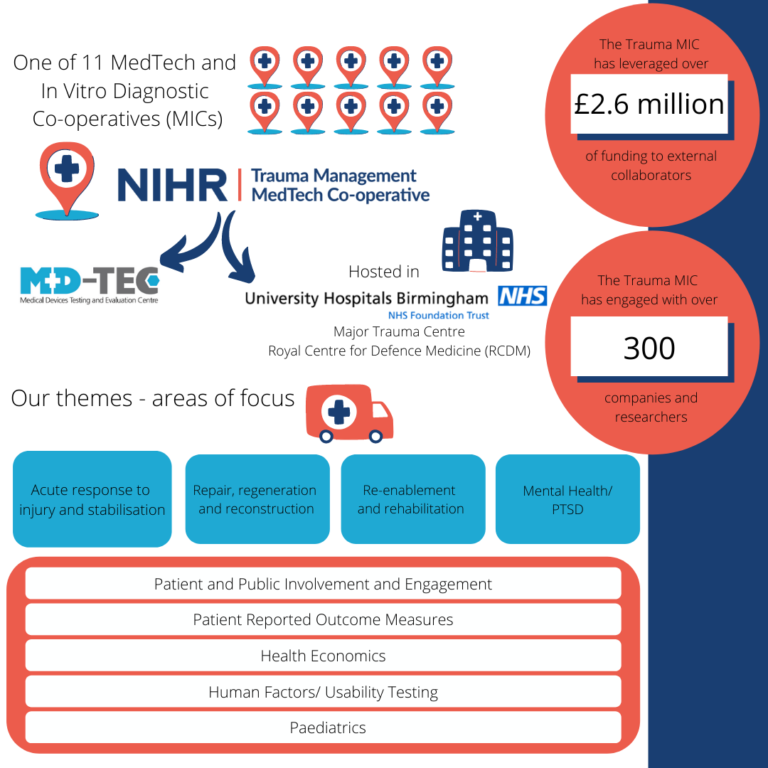
The NIHR Trauma Management MedTech Co-operative is one of eleven MedTech and In Vitro Diagnostic Co-operatives (MICs) set up to build expertise and capacity in the NHS to develop new medical technologies and provide evidence on commercially supplied in vitro diagnostic (IVD) tests.
The NIHR Trauma Management MedTech Co-operative is based at University Hospitals Birmingham NHS Foundation Trust (UHB). UHB is a Major Trauma Centre, treating the most serious casualties from across the West Midlands. UHB also hosts the Royal Centre for Defence Medicine (RCDM), which provides dedicated training for defence personnel and is a focus for medical research.
The remit of the Trauma Management MedTech Co-operative is to develop and deliver a programme of work that could improve clinical outcomes and quality of life for patients who have suffered a traumatic injury. The focus of our work will be on four distinct but inter-related parts of the patient pathway following traumatic injury:
1) Acute response to injury and stabilisation
The time between a traumatic injury and medical stabilisation is a crucial window for new developments. Improving decision support systems, monitoring, diagnostics and transport will all have major benefits to patients. In critical care, better infection control, management of inflammatory responses, better pain control, novel respiratory support, earlier detection of deterioration and maintenance of muscle mass are all areas where progress can be made.
2) Repair, Regeneration and Reconstruction
Following initial stabilisation there is the potential for significant advances in tissue repair, regeneration and reconstruction. These include tissue engineering, the management of burns and acute wounds, and surgical interventions.
3) Re-enablement and Rehabilitation
Survival following trauma can be the beginning of a long, intensive and demanding journey to recovery. Earlier and more effective rehabilitation will help patients’ recovery. There is potential for development in all areas of rehabilitation, including improved prosthetics, robotics, virtual reality and functional electrical muscle stimulation.
4) Mental Health
Individuals who have either experienced or witnessed a traumatic injury can suffer from Post Traumatic Stress Disorder (PTSD) and other mental health conditions. There is a role for SaMD, artificial intelligence, virtual reality and mixed reality in improving mental health.
What we do
Research
Act as a catalyst for the development of new medical devices, healthcare technologies and technology-dependent interventions in the NHS.
Healthcare
Improve the quality of life and effectiveness of healthcare services for trauma patients, from pre-hospital through to rehabilitation in the home.
Partnerships
Work collaboratively with patients and patient groups, charities, industry, clinicians and academics.
How we achieve it
IDENTIFY the “problem” and requirement for the development of device solutions
- Working with patients, carers, charities and healthcare professionals to identify unmet needs within the trauma field- with the aim to support research in those areas and share learning.
- Regulatory advice for companies wishing to attain CE and UK-CA approval for devices (this is required for devices to be used in a healthcare setting in the EU).
- Prototype development including 3D scanning.
- Usability studies – small scale studies designed to explore patients’/care professionals’ experiences of using a new device in order to achieve IEC 62366.
Provide ACCESS to Key People, resource and opportunities (Prioritising, Selection and Evaluation)
- ‘Matchmaking’ – finding appropriate resources and clinical/academic staff who would be willing to support projects with an external partner.
- Patient and Public Involvement expertise to gauge patients’ opinions and priorities relating to their condition, advice on public engagement, as well as PPI design support for studies.
- Grant award application support, either by directly applying for funding, collaborating on a project or providing consultation on the application process.
- Support to gain clinical evidence which can be used to substantiate the effectiveness and adoption claim.
SUPPORT Adoption (commercialisation and dissemination of device/healthcare technology)
- Events management for workshops and meetings with external partners to discuss new research opportunities.
- Dissemination of research and findings.
- Advice on market access and adoption.